Brief description
Catalytic oxidation is one of the fundamental reactions in chemical industry, which plays the crucial role in energy utilization, environmental protection and life health, and versatile intermediates in pharmaceuticals and fine chemicals are also popularly produced by catalytic oxidations. However, low efficiency in catalytic oxidation not only causes plenty loss of resources, but also causes seriously environmental pollution. Up to date, saving resources, reducing the energy and mass costs, and decreasing the pollutants have become the key concerns of our community, and they are also the key issues in the sustainable development of our society. Thereof, understanding the fundamental knowledges in catalytic oxidations, and next precisely controlling the catalysis are not only scientifically important, but also crucial for saving resources, decreasing the pollutants, and updating the on-going industrial processes. In our previous studies, we have investigated the oxidative relationships of active metal oxo and hydroxo species, and clarified how the oxidative properties of redox metal species changing with the changes of its physicochemical properties (Acc. Chem. Res., 2013, 46, 483-492), which has provided the guides for our coming catalyst design for oxidations.
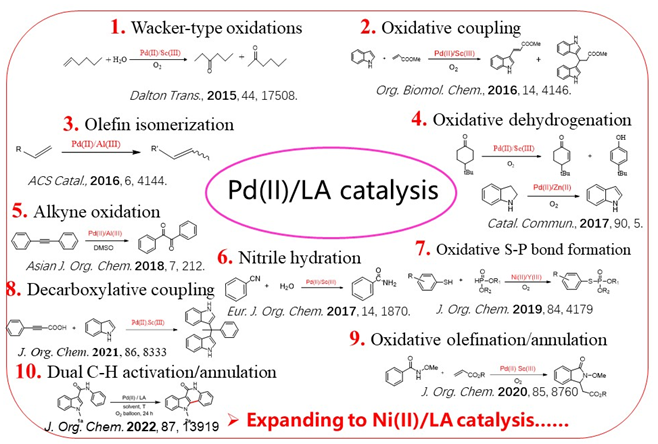
1) Organic synthesis by Pd(II)/LA catalysis
Wacker oxidation is a classic textbook example in homogeneous catalysis, in which the copper(II) cation is generally believed to play the key role in regeneration of the active Pd(II) species from the reduced Pd(0) after ethylene oxidation to acetaldehyde. In exploring Lewis acid promoted catalytic oxidations by redox metal complexes, we found that non-redox metal ions such as Sc3+ can promote Wacker-type oxidation much more efficient than Cu2+, implicating that the Lewis acid properties of the copper(II) cation may also play the significant role in Pd(II)-catalyzed Wacker-type oxidations as well as its redox properties (Dalton Trans., 2015, 44, 17508-17515). Inspired by this, we have defined a concept of Pd(II)/LA catalysis for organic synthesis, which has been applied a list of synthetic reactions. Particularly, using Pd(II)/LA as the platform, we have in-situ detected and fully characterized the agostic intermediate in C-H activation, and several other key intermediates have also been detected in C-H activation as well. Currently, new applications of Pd(II)/LA catalysis are still in exploration with C-H activation mechanism studies.
2) Lewis acid promoted dioxygen activation by transition metal complexes for catalysis
Enzymatic dioxygen activation and catalysis can proceed smoothly with high selectivity and efficiency at ambient temperature, whereas the catalytic oxidations in industry are generally performed at elevated temperature, which causes the happening of radical chain oxidation with low selectivity. The high activity of enzymatic oxidations is not only related to the dioxygen activation by the co-enzymes such as hemeiron, but also modulated by the hydrogen bond network during catalysis. However, such a moving hydrogen bong network cannot be efficiently mimicked by artificial redox catalysts. Fundamentally, the hydrogen bond interaction is one category of electrostatic interaction; inspired by this understanding, we are exploring Lewis acid promoted dioxygen activation by redox metal complexes for catalysis (Enzymatic Brösted acid (that is, hydrogen bond) vs Lewis acid). The electrostatic interaction between Lewis acid and the superoxide generated by dioxygen activation with transition metal complexes can stabilize the superoxide species, driving the equilibrium of dioxygen activation toward the superoxide formation, meanwhile improving the electrophilic capability of the superoxide, thus driving the catalytic oxidation proceeding.

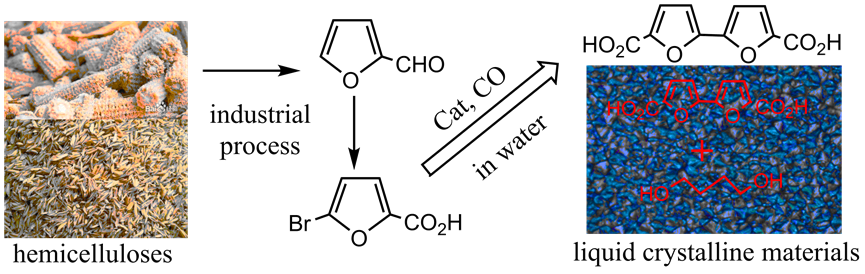
3) Exploring novel catalytic technologies to derive monomers from bioresources
With the rapid depletion of the fossil resources, its replacements by renewable biomass as the carbon sources of chemical industry have been the hot topic in academic and industrial communities. Among versatile polysaccharides, C6 based ones such as glucose and fructose receive most attentions, especially C6 derived HMF and its derivatives have been well explored, while C5 based polysaccharides received less attentions. Compared with C6 based HMF, C5 based furfural is on-going produced in large scale from agricultural and forestry byproducts. In view of the large availability of its raw materials and easy expanding of its industrial production, using furfural as the platform to obtain valorized chemicals is great attractive. Based on this understanding, we are exploring novel catalytic technologies to transform furfural to maleic acid and maleic anhydride through oxidations, to transform furfural derived 5-bromofuroic acid to 2,5-furandicarboxylic acid and 2,2-bifuryl-5,5-dicarboxylic acid, etc., to provide bio-based new monomers for polymer industry.
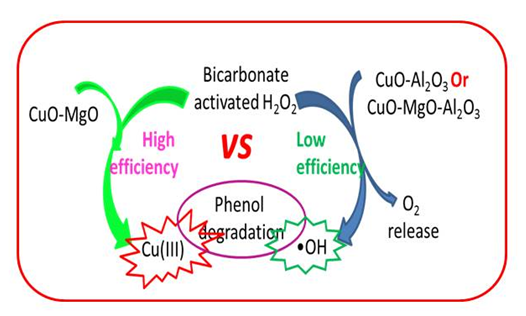
4) Exploring novel catalytic technologies for wastewater treatment
The lacking of water resource has been the global challenges for a long while. Although versatile technologies have been explored to recycle the polluted water, the on-going technologies are apparently not enough to overcome the challenges in wastewater treatment. Among versatile chemical technologies, supported metal oxide catalysts as the advanced oxidation technology receive much attention, however, it still faces great challenges. For example, with the wastewater treatment proceeding, the aqueous medium is gradually acidified, which may cause the redox metal ions leaching from the support, leading to shorten lifetime of the catalyst with potential heavy metal pollution. Our solution is to explore the alkaline catalytic technology for wastewater treatment, in which the wastewater treatment is performed und weakly alkaline conditions that can efficiently prevent the metal ion leaching from the support.